
1、你的整個訓練、減脂、營養,安排,都有問題
2、其中問題最大的,是你的睡眠
一、睡眠問題,這個作息是在拿自己的生命/健康開玩笑
眾所周知,人類是日落而息的,晚上6-8點以後就不應該從事相對劇烈的活動了。
強行訓練,打破自然規律,導致神經過分興奮,晚上睡不著,無異於慢性自殺。
我覺得是常識,為什麽題主會不清楚呢。
口說無憑,我們還是看證據吧。
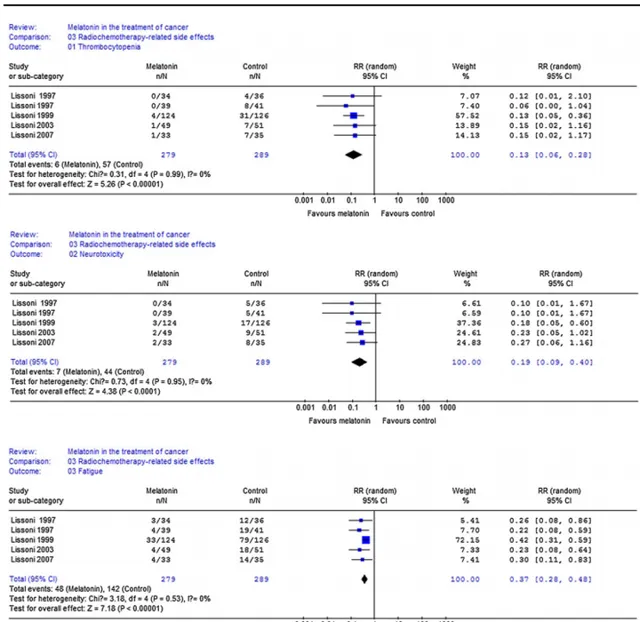
1、Kakizaki等人針對22320名日本人的研究發現,睡眠充足者發生前列腺癌的機率降低了52%[2];

2、Tatsuhiko等人針對14052人的研究發現[3],不規律作息(輪班,一會白班一會夜班)工作者前列腺癌的發病率提高200%;與之相對的是,規律作息(固定夜班)者的相關風險增加很少且不顯著;
3、Liu等人在日本的進行的一項針對幾百人的研究發現[1],每周工作60小時、或每日睡眠少於5小時,急性心肌梗死的發作率大振幅提高。
4、輪班工人的晝夜節律失調與心臟代謝異常[15]、代謝症候群、心血管疾病的風險增加有關[16,17,18,19]。
5、睡眠不足增減少褪黑激素的產生,擾亂晝夜節律[48],增加冠心病風險[49]、2型糖尿病風險[50]、肥胖風險[51]、抑郁癥風險[52],從而增加死亡風險[53]。
值得一提的是,睡眠不足與癌癥的關聯,與褪黑素有密切關系[4,5,6]。
褪黑素它調節性激素的分泌[8,9],對前列腺癌和乳癌細胞,具有抗增殖作用[6]。
中國有些研究實驗室環境中反復論證了褪黑素具有一定抗癌的作用,提倡應當在癌癥治療中作為一種輔助手段[92]。
此外,褪黑素還有維持免疫系統功能、保護DNA、抗氧化、血糖調節等功能[10]。
由於褪黑素主要在睡眠中分泌,睡眠不足會減少它的分泌[7]。
但是,我並不建議你們自己去吃褪黑素。
二、睡眠不足對增肌/減脂的顯著抑制
我有14年的系統訓練史,最大體重超過100KG。
在我精力充沛不太工作的時候,可以坐姿下半程100KG杠鈴推舉做組,4-5次組——自由重量。
根據我的個人經驗:
當然,這只是我的個人經驗,凡事講證據。
1、2020年,Rein等人研究了一群人,把他們分為2組:
A組:訓練+睡眠最佳化(10人);
B組:訓練+不最佳化睡眠組(12人)

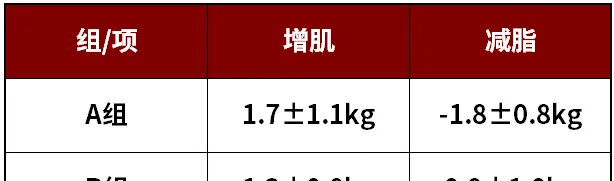
也就是說,睡眠不足會抑制增肌、抑制減脂,讓人又胖肌肉又少。
當然, 上面一個研究還相對溫和,下面一個更典型 。
三、睡眠不足對減脂的不利
自1980年以來,全球肥胖患病率翻了一倍以上[11],肥胖的流行與睡眠時間縮短的趨勢是吻合的[12]。
越來越多的證據表明,睡眠不足,會促進肥胖及其並行癥發生[13,14];對於中老年人來說,睡眠時間較短導致他們的肥胖風險增加52%[21];
有趣的是,對於睡眠不足5小時的人來說,每多睡一小時,BMI就會降低0.35[20],這相當於一個1.7米身高的人瘦了1-1.5kg左右。
請註意前提:啥都不做,只是多睡覺,就會瘦。
(在我的個人經驗中,瘦臉與睡眠關系特別大,連續睡得好我的臉就不腫)
總的來說,大量證據都證明了睡眠不足會導致肥胖。
四、 睡眠不足會促進食欲,讓人多吃
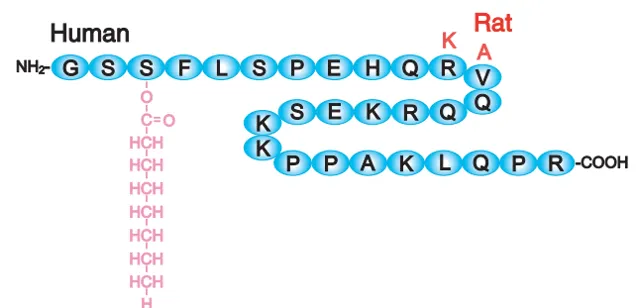
人體內有一種激素,叫做 ghrelin ,它是一種胃腸分泌的肽類物質[97,98]。
肽,有些朋友不熟悉,沒關系,至少大家都知道蛋白質:大量胺基酸由鍵連線在一起所組成的;其實,肽,無非就是少量的胺基酸連線在一起,可以粗略的這樣理解。
ghrelin,有人給他起了中文名叫加拉寧神經肽,它由28個胺基酸連結而成。人和老鼠的ghrelin類似,差不多長這樣:
ghrelin由胃腸細胞分泌。這些細胞在高倍放大下長這樣。
胃腸細胞分泌ghrelin後,由血液迴圈,到達下丘腦, 它會刺激下丘腦的弓核區域,從而刺激食欲 ,所以ghrelin被認為是一種刺激食欲、調劑能量平衡的激素[93,94,95,96]。
用通俗的話來說,它的作用是讓你餓,讓你吃,讓你朝胖的方向發展,所以它也叫促饑餓素,較高水平的ghrelin有助於脂肪的保留,抑制脂肪分解[36,37,38]。
與之對應的,還有抑制食欲的激素leptin—瘦素[44],瘦素既能抑制食欲,也能增加能量消耗[27]。
大量研究發現[13,22,23,24,25],睡眠不足,ghrelin會被上調,leptin會被抑制,人傾向於吃更多東西,所以就容易胖。
Spiegel等人[26]分析了健康年輕男性的睡眠,提供了食欲素系統的示意圖,它表示睡眠和進食之間的聯系。一些促進食欲的神經元會調節下丘腦攝食中心,產生「進食獎賞」[22]。
也就是說,睡不好的人容易吃的更多,所以更容易胖。
五、睡眠不足還會降低代謝,減少熱量消耗
從道理上說,睡不好,就不想動,沒精神。
因此,證據顯示,睡眠不足,身體會自發的減少運動,在白天導致嗜睡和疲勞,增加久坐行為,從而減少與運動相關的能量消耗[28];
睡眠不足還減少白天安靜狀態的能量消耗[42,43,44,45,46],減少能量支出[47],例如透過降低體溫的方式[29](產熱也需要耗能);
雖然有些研究發現睡眠不足導致夜間能量消耗增加了32%[30],但這被更多的進食所抵消了,因為睡眠不足上調了促進食欲的激素ghrelin、下調了抑制食欲的激素leptin[31,32];
例如Nedeltcheva等人[33]觀察了10名超重中年人發現,睡眠不足導致ghrelin水平糊饑餓感增加;
Brondel等人[34]發現12名體重正常的年輕人在夜間睡眠4小時後,熱量攝入增加、饑餓感增強;
Tasali等人[35]對10名健康年輕人觀察發現,與8.5小時相比,連續4天晚上只4.5小時,熱量攝入增加了14%。
六、 睡眠不足還抑制脂肪分解
睡不夠,對於正在進行節食、制造熱量缺口的人來說很不利。
Arlet等人證實,對於節食的人來說,睡眠充足者(8.5小時,空心三角)比睡眠不足者(5.5小時,實心圓)的脂肪燃燒率更高。
下圖中的RQ,是呼吸商,是一種呼吸測量技術[100]。
這種技術能透過分析能從我們呼吸的氣體中來分析我們的身體是傾向於燃燒碳水供能還是燃燒脂肪供能更多[99,101]。
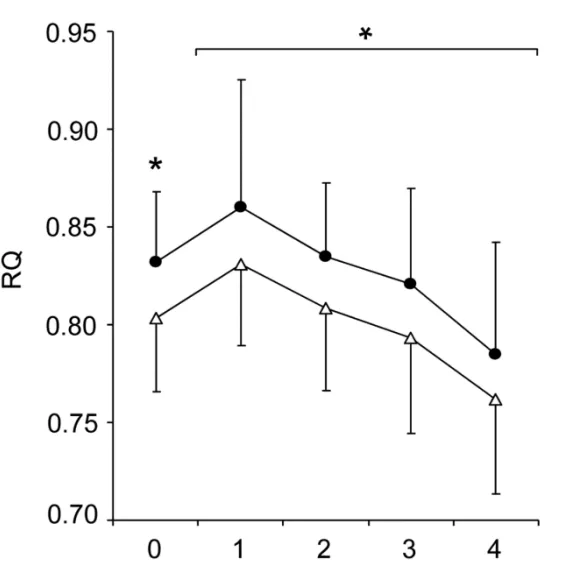
呼吸商越低表示燃燒脂肪越多,越接近1表示燃燒碳水更多。
透過這種技術,Arlet等人證實:
對於正在節食試圖減脂的人來說,睡眠不足會大大的降低我們的減脂效果。
在Arlet等人發現,同樣面臨熱量不足,但是睡眠不足的人減脂的量,比睡眠充足的人減少了 56%之多 [39],這也和Krotkiewski等人[40]、Rein等人的研究結論相支持。
所以,我們回過頭來看,為什麽題主減脂好像減不動,那就完全正常了。
七、睡眠不足,減肌
人和動物的肌肉,不是固定不動的,是在不斷和成和分解,不斷變化的。
生命的基本特征是新陳代謝[60],不斷更新組織:舊的組織不斷死亡(被分解)[61],新的組織不斷產生(被合成)。
人體肌纖維中,有一些更細的結構,叫肌原纖維-myofibrils(下圖中的綠框)。
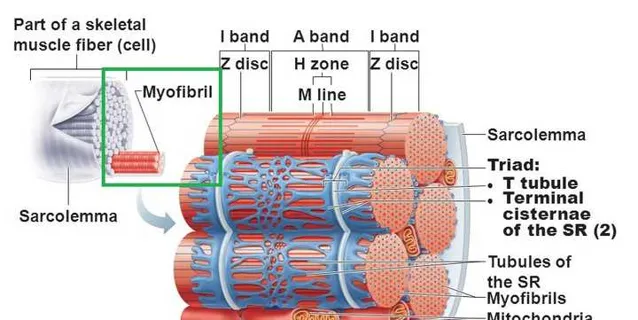
肌原纖維的內部是肌蛋白[54,55];
愛好者們經常說,你增肌了,或者你肌肉圍度掉了,其實這就是因為肌原纖維中的蛋白質增加了[56]、或者減少了[57]。
當然,肌原纖維中的蛋白質有很多種[58,59],我們不鋪開說,在這就用一個「肌蛋白」來統稱。
我們的肌肉中, 肌肉大小變化取決於肌肉中的蛋白質分解和合成的差值 [62,108,109]。
在常態下,它們不斷被分解、不斷被合成,維持平衡,我們的肌肉大小就不變。
合成>分解=正平衡=增肌=肌纖維變粗
分解>合成=負平衡=減肌=肌纖維變細
這些內容跟本文的關系在於:睡眠不足,都跟肌肉的「凈平衡」有關。
有許多證據表明,睡眠於維持肌肉的量相當重要[103,104],在睡眠<7小時每晚、或自稱睡眠質素較低的人中,骨骼肌量下降、骨骼肌流失相當較為常見[105,106,107]。
在持續2周,每晚5.5小時的睡眠剝奪研究中,人類也表現出了大振幅的骨骼肌流失[90]。
上個世紀80年代就已經證明了[110],連續3天睡眠不足,骨骼肌就會明顯加速流失(尿液中的氮排放量)顯著增加。
八、 睡眠不足升高皮質醇水平
我們在上面剛剛講了,肌肉大小是個動態平衡過程,取決於肌肉的合成與分解之間的差值。
我們從一些數據看到,睡眠不足者的肌肉量和肌肉質素都較低[90,91],睡眠不足是導致肌肉萎縮的重要因素之一[64]。
因為,睡眠不足會導致皮質醇水平升高[63,65]。
而皮質醇的作用剛好就是抑制肌肉合成、促進肌肉分解[66]。
William等人測試了近140民遊牧民族男子發現,皮質醇水平越高,肌肉橫截面積越小,兩者呈負相關關系[102]。
九、 睡眠不足擾亂合成代謝激素的分泌
在睡眠剝奪的研究中,個體的分解代謝激素(如皮質醇)被上調、合成代謝激素(如睪酮、IGF-1)等被下調[67,68]。
尤其是IGF-1,它是非常重要的促進肌肉增長的激素[69,70,71,72,73,74];
IGF-1對於維持肌肉的能量代謝[80、81]、對於肌蛋白合成極為重要[75,76,77,78],甚至於有些研究者認為它是必不可少的[79]。
這些,我們在之前的文章中已經寫過,就不贅述了。
生長激素到底能夠增加肌纖維數量嗎?
十、 睡眠不足促成胰島素抵抗
我們在之前的文章中也說過,胰島素是一種重要的合成代謝激素。
既然只有蛋白質才能轉化肌肉為什麽增肌期間還要攝入大量碳水?
胰島素同時促進脂肪、碳水、胺基酸的吸收,非常有助於增肌[82,83]。
至於為什麽胰島素能增肌,我們在之前的文章中也解釋過,這裏稍微復習一下:
胰島素增肌的路徑是首先啟用IRS-1和2,然後按順序啟用PI3K-Akt-mTOR-S6K,最後刺激DNA轉錄[84,85,86,87];
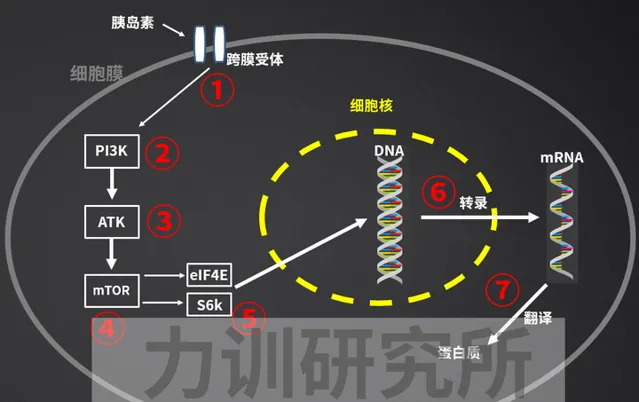
對本文來說,睡眠不足可促進胰島素抵抗[88],抵消胰島素促進DNA轉錄(合成肌蛋白)的作用,從而限制肌肉增長[89]。
所以,我們能看到有些支持性數據顯示,1-2個晚上的完全睡眠剝奪,導致24小時的尿氮排泄的增加。
眾所周知,蛋白質分解是人體腦葉排出氮的來源,一般每6.25g蛋白質含有1g的氮。
所以,尿液中氮出量的增加,意味著著肌肉的分解增加,肌肉的凈變化偏向於損失。
十一、晚上睡得少,盡量白天補一些
訓練到一定強度的朋友都知道,如果要保持高強度的訓練,比如深蹲在150-180KG做組、臥推100KG十幾個一組的水平上,晚上8小時左右是不夠的。
這種情況下,大家都感覺到中午的睡眠至關重要:全靠中午午睡來「回血」,以支撐下午下班後的訓練。
當然,上述只是民間經驗,其實科學也證實了這一點。
Water等人進行了研究[111],他讓受試者晚上只睡4個小時(23:00-3:00),然後讓他們在午後13:00-13:30可以睡半小時(打盹組)或者是不睡(對照組)。
打盹組的沖刺時間得到了改善,平均2米沖刺時間從1.060秒下降到1.019秒;20米短跑的平均時間從3.971s下降到3.878s。
結果表明午後小睡可以提高部份睡眠不足後精神狀態和和運動表現。
小結:
1、訓練不宜在晚上進行,如果影響了睡眠,得不償失,很傷
2、睡不夠,增肌、減脂的效果都大打折扣
3、睡眠的重要性,以我個人經驗而言,淩駕於訓練和飲食之上
4、如果夜間睡不夠,白天午睡至關重要
5、減脂不能一味低碳,低碳減脂不合理也不科學,數據很多,以後有空講
推薦閱讀:
有哪些是你健身久了知道的事?
肉崽:力訓研究所課程介紹
肉崽:力訓研究所線上期刊介紹
References
1. Y Liu, H Tanaka, The Fukuoka Heart Study Group.Overtime work, insufficient sleep, and risk of non-fatal acute myocardial infarction in Japanese men.Occup Environ Med 2002;59:447–451.
2. Kakizaki M, Inoue K, Kuriyama S, et al. Sleep duration and the risk of prostate cancer: the Ohsaki Cohort Study. Br J Cancer. 2008;99:176–8.
3. Tatsuhiko Kubo 1 , Kotaro Ozasa, Kazuya Mikami, Kenji Wakai, Yoshihisa Fujino, Yoshiyuki Watanabe, Tsuneharu Miki, Masahiro Nakao, Kyohei Hayashi, Koji Suzuki, Mitsuru Mori, Masakazu Washio, Fumio Sakauchi, Yoshinori Ito,
Takesumi Yoshimura, Akiko Tamakoshi.Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan collaborative cohort study.Am J Epidemiol. 2006 Sep 15;164(6):549-55.
4. Brzezinski A (1997) Melatonin in humans. N Engl J Med 336: 186 – 195 Conlon M, Lightfoot N, Kreiger N (2007) Rotating shift work and risk of prostate cancer. Epidemiology 18: 182 – 183
5. Schernhammer ES, Schulmeister K (2004) Melatonin and cancer risk: does light at night compromise physiologic cancer protection by lowering serum melatonin levels? Br J Cancer 90: 941 – 943
6. Shiu SY (2007) Towards rational and evidence-based use of melatonin in prostate cancer prevention and treatment. J Pineal Res 43: 1–9
7. Wehr TA (1991) The durations of human melatonin secretion and sleep respond to changes in daylength (photoperiod). J Clin Endocrinol Metab 73: 1276 – 1280
8. Martin JE, Klein DC (1976) Melatonin inhibition of the neonatal pituitary response to luteinizing hormone-releasing factor. Science 191: 301 – 302
9. Aleandri V, Spina V, Morini A (1996) The pineal gland and reproduction. Hum Reprod Update 2: 225 – 235
10. B Claustrat, J Leston Melatonin: Physiological effects in humans.Neurochirurgie. Apr-Jun 2015;61(2-3):77-84. doi: 10.1016/j.neuchi.2015.03.002. Epub 2015 Apr 20.
11. Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011; 377:557–567.
12. CDC. Unhealthy sleep-related behaviors – 12 States, 2009. MMWR Morb Mortal Wkly Rep. 2011; 60:233–238.
13. Morselli L, Leproult R, Balbo M, Spiegel K. Role of sleep duration in the regulation of glucose metabolism and appetite. Best Pract Res Clin Endocrinol Metab. 2010; 24:687–702.
14. Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab. 2010; 24:731–743.
15. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009; 106:4453–4458.
16. Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010; 330:1349–1354.
17. Esquirol Y, Bongard V, Mabile L, et al. Shift work and metabolic syndrome: respective impacts of job strain, physical activity, and dietary rhythms. Chronobiol Int. 2009; 26:544–559.
18. De Bacquer D, Van Risseghem M, Clays E, et al. Rotating shift work and the metabolic syndrome: a prospective study. Int J Epidemiol. 2009; 38:848–854.
19. Garaulet M, Ordovas JM, Madrid JA. The chronobiology, etiology and patho-physiology of obesity. Int J Obes (Lond). 2010; 34:1667–1683.
20. Cappuccio, F.; Miller, M. The epidemiology of sleep and cardiovascular risk and disease.. In: Cappuccio, F.; Miller, M.; Lockley, S., editors. Sleep, health and society: from aetiology to public health. Oxford University Press; Oxford: 2010. p. 83-110.
21. Magee CA, Caputi P, Iverson DC. Is sleep duration associated with obesity in older Australian adults? J Aging Health. 2010; 22:1235–1255.
22. Pannain S, Miller A, Van Cauter E. Sleep loss, obesity and diabetes: prevalence, association and emerging evidence for causation. Obes Metab-Milan. 2008; 4:28–41.
23. Spiegel K, Leproult R, L'Hermite-Baleriaux M, et al. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004; 89:5762–5771.
24. Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004; 141:846–850.
25. Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008; 9(Suppl 1):S23–S28.
26. Spiegel K, Tasali E, Leproult R, et al. Twenty-four-hour profiles of acylated and total ghrelin: relationship with glucose levels and impact of time of day and sleep. J Clin Endocrinol Metab. 2011; 96:486–493.
27. Scarpace PJ, Matheny M, Pollock BH, Tumer N. Leptin increases uncoupling protein expression and energy expenditure. Am J Physiol. 1997; 273(1 Pt 1):E226–E230.
28. Schmid SM, Hallschmid M, Jauch-Chara K, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009; 90:1476–1482.
29. Vaara J, Kyrolainen H, Koivu M, et al. The effect of 60-h sleep deprivation on cardiovascular regulation and body temperature. Eur J Appl Physiol. 2009; 105:439–444.
30. Jung CM, Melanson EL, Frydendall EJ, et al. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011; 589(Pt 1):235–244.
31. Mullington JM, Chan JL, Van Dongen HP, et al. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003; 15:851–854.
32. Schmid SM, Hallschmid M, Jauch-Chara K, et al. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J Sleep Res. 2008; 17:331–334.
33. Nedeltcheva AV, Kilkus JM, Imperial J, et al. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010; 153:435–441.
34. Brondel L, Romer MA, Nougues PM, et al. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010; 91:1550–1559.
35. Tasali E, Broussard J, Day A, et al. Sleep curtailment in healthy young adults is associated with increased ad lib food intake [meeting abstract]. Sleep. 2009; 32(Suppl):A163.
36. Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000; 407(6806): 908–913.
37. Nogueiras R, Tschop MH, Zigman JM. Central nervous system regulation of energy metabolism: ghrelin versus leptin. Ann N Y Acad Sci. 2008; 1126:14–19.
38. Rodriguez A, Gomez-Ambrosi J, Catalan V, et al. Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int J Obes (Lond). 2009; 33(5):541–552.
39. Arlet V. Nedeltcheva, MD1, Jennifer M. Kilkus, MS2, Jacqueline Imperial, RN2, Dale A. Schoeller, PhD3, and Plamen D. Penev, MD, PhD.Insufficient sleep undermines dietary efforts to reduce adiposity.Ann Intern Med. 2010 October 5; 153(7): 435–441.
40. Krotkiewski M, Landin K, Mellstrom D, Tolli J. Loss of total body potassium during rapid weight loss does not depend on the decrease of potassium concentration in muscles. Different methods to evaluate body composition during a low energy diet. Int J Obes Relat Metab Disord. 2000; 24(1):101–107.
41. Scrimshaw NS, Habicht JP, Pellet P, Piche ML, Cholakos B. Effects of sleep deprivation and reversal of diurnal activity on protein metabolism of young men. Am J Clin Nutr. 1966; 19(5): 313–319.
42. Ravussin E, Burnand B, Schutz Y, Jequier E. Energy expenditure before and during energy restriction in obese patients. Am J Clin Nutr. 1985; 41(4):753–759.
43. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. New England Journal of Medicine. 1995; 332(10):621–628.
44. Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005; 115(12):3579–3586.
45. Landsberg L. Feast or famine: the sympathetic nervous system response to nutrient intake. Cell Mol Neurobiol. 2006; 26(4–6):497–508.
46. Redman LM, Heilbronn LK, Martin CK, et al. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One. 2009; 4(2):e4377.
47. Schmid SM, Hallschmid M, Jauch-Chara K, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009; 90(6):1476–1482.
48. Medic G., Wille M., Hemels M.E. Short- and long-term health consequences of sleep disruption. Nat. Sci. Sleep. 2017;9:151–161.
49. Lao X.Q., Liu X., Deng H., Chan T., Ho K.F., Wang F., Vermeulen R., Tam T., Wong M.C., Tse L.A., et al. Sleep quality, sleep duration, and the risk of coronary heart disease: A prospective cohort study with 60, 586 adults. J. Clin. Sleep Med. 2018;14:109–117.
50. Lou P., Chen P., Zhang L., Zhang P., Yu J., Zhang N. Relation of sleep quality and sleep duration to type 2 diabetes: A population-based cross-pal survey. BMJ. 2012;2:e000956.
51. Nedeltcheva A.V., Scheer F.A. Metabolic effects of sleep disruption, links to obesity and diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2014;21:293–298.
52. Leary K.O., Bylsma L.M., Rottenberg J., Leary K.O., Bylsma L.M., Why J.R. Why might poor sleep quality lead to depression? A role for emotion regulation regulation. Cogn. Emot. 2016;31:1698–1706.
53. Crowley K. Sleep and sleep disorders in older adults. Neuropsychol. Rev. 2011;21:41–53.
54. N Narayanan, J Eapen.Protein synthesis by rat cardiac muscle myofibrils.Biochim Biophys Acta. 1973 Jun 23;312(2):413-25.
55. Kono,Fumiaki,Shimamoto,Yuta,Ishiwata,Shin'ichi.1P246 Effect of lattice spacing on SPOC of skeletal myofibrils(8. Muscle contraction and muscle protein,Poster Session,Abstract,Meeting Program of EABS & BSJ 2006)
56. D L, Pak-Loduca, K A Obert, KE Yarasheski.Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78-84 and 23-32 yr olds.Am J Physiol Endocrinol Metab. 2000 Apr;278(4):E620-6.
57. Ruowei, Li.The temporal control of load-dependent anabolic and catabolic pathways in human extensor muscles.Manchester Metropolitan University
58. S Winegrad.Myosin-Binding Protein C (MyBP-C) in Cardiac Muscle and Contractility.Molecular and Cellular Aspects of Muscle Contraction.
59. C M Rembold, R A Murphy.Myoplasmic calcium, myosin phosphorylation, and regulation of the crossbridge cycle in swine arterial smooth muscle.Circ Res. 1986 Jun;58(6):803-15.
60. SAS Gropper,JL Smith,J Groff.Advanced Nutrition and Human Metabolism.GROPPER, Sareen Annora Stepnick, Jack L. SMITH and James L. GROFF. Advanced nutrition and human metabolism. 5th ed. United States: Wadsworth/Cengage Learning, 2009. xvii, 600. ISBN 9780495116578.
61. T P Stein, M D Schluter.Human skeletal muscle protein breakdown during spaceflight.Am J Physiol. 1997 Apr;272(4 Pt 1):E688-95.
62. Atherton PJ, Phillips BE, Wilkinson DJ.Exercise and Regulation of Protein Metabolism.Prog Mol Biol Transl Sci. 2015;135:75–98.
63. Banks S., Dinges D.F. Behavioral and physiological consequences of sleep restriction. J. Clin. Sleep Med. 2007;3:519–528.
64. Piovezan R.D., Abucham J., dos Santos R.V.T., Mello M.T., Tufik S., Poyares D. The impact of sleep on age-related sarcopenia: Possible connections and clinical implications. Ageing Res. Rev. 2015;23:210–220.
65. Goodin B.R., Smith M.T., Quinn N.B., King C.D., McGuire L. Poor sleep quality and exaggerated salivary cortisol reactivity to the cold pressor task predict greater acute pain severity in a non-clinical sample. Biol. Psychol. 2012;91:36–41.
66. Peeters G.M.E.E., Van Schoor N.M., Van Rossum E.F.C., Visser M., Lips P.T.A.M. The relationship between cortisol, muscle mass and muscle strength in older persons and the role of genetic variations in the glucocorticoid receptor. Clin. Endocrinol. (Oxf.) 2008;69:673–682.
67. Everson CA, Crowley WR.Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats. Am J Physiol Endocrinol Metab. 2004 Jun; 286(6):E1060-70.
68. von Treuer K, Norman TR, Armstrong SM. Overnight human plasma melatonin, cortisol, prolactin, TSH, under conditions of normal sleep, sleep deprivation, and sleep recovery. J Pineal Res.
69. Tadashi Yoshida1,Patrice Delafontaine.Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy.Cells. 2020 Sep;9(9).
70. Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75: 73– 82, 1993.
71. Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev 17:481–517, 1996.
72. Galvin CD, Hardiman O, Nolan CM. IGF-1 receptor mediates differentiation of primary cultures of mouse skeletal myoblasts. Mol Cell Endocrinol 200: 19 –29, 2003.
73. Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I(Igf-1) and type 1 IGF receptor (Igf1r). Cell 75: 59 –72, 1993.
74. Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, PittsMeek S, Dalton D, Gillett N, Stewart TA. IGF-I is required for normal embryonic growth in mice. Gene Dev 7: 2609 –2617, 1993.
75. Rommel C., Bodine S.C., Clarke B.A., Rossman R., Nunez L., Stitt T.N., Yancopoulos G.D., Glass D.J. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 2001;3:1009–1013.
76. Peng X.D., Xu P.Z., Chen M.L., Hahn-Windgassen A., Skeen J., Jacobs J., Sundararajan D., Chen W.S., Crawford S.E., Coleman K.G., et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17:1352–1365.
77. Bodine S.C., Latres E., Baumhueter S., Lai V.K., Nunez L., Clarke B.A., Poueymirou W.T., Panaro F.J., Na E., Dharmarajan K., et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708.
78. Pallafacchina G., Calabria E., Serrano A.L., Kalhovde J.M., Schiaffino S. A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc. Natl. Acad. Sci. USA. 2002;99:9213–9218.
79. Glass D.J. Molecular mechanisms modulating muscle mass. Trends Mol. Med. 2003;9:344–350.
80. Bassil MS, Gougeon R. Muscle protein anabolism in type 2 diabetes. Curr Opin Clin Nutr Metab Care. 2013;16:83–88.
81. Morley JE. Diabetes, sarcopenia, and frailty. Clin Geriatr Med. 2008;24:455–469.
82. Biolo G, Declan R, and Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest 95: 811–819, 1995
83. Biolo G, Williams BD, Declan Fleming RY, and Wolfe RR. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes 48: 949– 957, 1999.
84. Hillier T, Long W, Jahn L, Wei L, and Barrett EJ. Physiological hyperinsulinemia stimulates p70S6k phosphorylation in human skeletal muscle. J Clin Endocrinol Metab 85: 4900–4904, 2000.
85. Farrell PA, Hernandez JM, Fedele MJ, Vary TC, Kimball SR, and Jefferson LS. Eukaryotic initiation factors and protein synthesis after resistance exercise in rats. J Appl Physiol 88: 1036–1042, 2000.
86. Fluckey JD, Vary TC, Jefferson LS, Evans WJ, and Farrell PA. Insulin stimulation of protein synthesis in rat skeletal muscle following resistance exercise is maintained with advancing age. J Gerontol Biol Sci 51A: B323–B330, 1996.
87. Verdu J, Buratovich MA, Wilder EL, and Birnbaum MJ. Cell-autonomous regulation of cell and organ growth in Drosophila by Akt/PKB. Nature Cell Biol 1: 500–506, 1999.
88. Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435–441.
89. Kant GJ, Genser SG, Thorne DR, Pfalser JL, Mougey EH. Effects of 72 hour sleep deprivation on urinary cortisol and indices of metabolism. Sleep. 1984;7:142–146.
90. Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435–441.
91. Kant GJ, Genser SG, Thorne DR, Pfalser JL, Mougey EH. Effects of 72 hour sleep deprivation on urinary cortisol and indices of metabolism. Sleep. 1984;7:142–146.
92. Ye-min Wang. Bao-zhe Jin. Fang Ai. Chang-hong Duan. Yi-zhong Lu. Ting-fang Dong. Qing-lin Fu.The eYcacy and safety of melatonin in concurrent chemotherapy or radiotherapy for solid tumors: a meta-analysis of randomized controlled trials.
93. van der Lely AJ. 2009. Ghrelin and new metabolic frontiers. Horm Res 71(Suppl 1):129–133
94. Gasco V, Beccuti G, Marotta F, Benso A, Granata R, Broglio F, Ghigo E. 2010. Endocrine and metabolic actions of ghrelin. Endocr Dev 17:86–95
95. Granata R, Baragli A, Settanni F, Scarlatti F, Ghigo E. 2010. Unraveling the role of the ghrelin gene peptides in the endocrine pancreas. J Mol Endocrinol 45:107–118
96. Lim CT, Kola B, Korbonits M, Grossman AB. 2010. Ghrelin's role as a major regulator of appetite and its other functions in neuroendocrinology. Prog Brain Res 182:189–205
97. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. 1999. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660
98. Masayasu Kojima, Kenji Kangawa.Ghrelin: structure and function.Physiol Rev. 2005 Apr;85(2):495-522.
99. Schutz Y. Abnormalities of fuel utilization as predisposing to the development of obesity in humans. Obes Res. (1995) 3 (Suppl. 2):173S−8S.
100. Respiratory Quotient.978-3-540-36065-0.
101. L.GARBY, A.ASTRUP.The relationship between the respiratory quotient and the energy equivalent of oxygen during simultaneous glucose and lipid oxidation and lipogenesis.First published: March 1987
102. William D Lukas, Benjamin C Campbell, Kenneth L Campbell.Urinary cortisol and muscle mass in Turkana men.Am J Hum Biol. Jul-Aug 2005;17(4):489-95.
103. Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA & Penev PD (2010). Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med 153, 435–441.
104. Dattilo M, Antunes HK, Medeiros A, Monico‐Neto M, Souza Hde S, Lee KS, Tufik S & de Mello MT (2012). Paradoxical sleep deprivation induces muscle atrophy. Muscle Nerve 45, 431–433.
105. Chien MY, Wang LY & Chen HC (2015). The relationship of sleep duration with obesity and sarcopenia in community‐dwelling older adults. Gerontology 61, 399–406.
106. Buchmann N, Spira D, Norman K, Demuth I, Eckardt R & Steinhagen‐Thiessen E (2016). Sleep, muscle mass and muscle function in older people. Dtsch Arztebl Int 113, 253–260.
107. Hu X, Jiang J, Wang H, Zhang L, Dong B & Yang M (2017). Association between sleep duration and sarcopenia among community‐dwelling older adults: a cross‐pal study. Medicine (Baltimore) 96, e6268.
108. Rennie MJ (1985). Muscle protein turnover and the wasting due to injury and disease. Br Med Bull 41, 257–264.
109. Gibson JN, Halliday D, Morrison WL, Stoward PJ, Hornsby GA, Watt PW, Murdoch G & Rennie MJ (1987). Decrease in human quadriceps muscle protein turnover consequent upon leg immobilization. Clin Sci (Lond) 72, 503–509.
110. Kant GJ, Genser SG, Thorne DR, Pfalser JL & Mougey EH (1984). Effects of 72 h sleep deprivation on urinary cortisol and indices of metabolism. Sleep 7, 142–146.
111. J Waterhouse, G Atkinson, B Edwards, T Reilly The role of a short post-lunch nap in improving cognitive, motor, and sprint performance in participants with partial sleep deprivation.J Sports Sci. 2007 Dec;25(14):1557-66.
112. Amber Brooks, Leon Lack.A brief afternoon nap following nocturnal sleep restriction: which nap duration is most recuperative? Sleep. 2006 Jun;29(6):831-40.











