科学足迹出版社供稿:科学足迹出版社(SFP)
期刊 :Science Advances
期刊 :科学进展
题目
:Full-nitro-nitroamino cooperative action: Climbing the energy peak of benzenes with enhanced chemical stability
题目 :全硝基硝氨基协同作用:攀登苯的能量峰,增强化学稳定性
作者 :Qi Sun, Ning Ding, Chaofeng Zhao, Qi Zhang, Shaowen Zhang, Shenghua Li, Siping Pang
摘要 :More nitro groups accord benzenes with higher energy but lower chemical stability. Hexanitrobenzene (HNB) with a fully nitrated structure has stood as the energy peak of organic explosives since 1966, but it is very unstable and even decomposes in moist air. To increase the energy limit and strike a balance between energy and chemical stability, we propose an interval full-nitro-nitroamino cooperative strategy to present a new fully nitrated benzene, 1,3,5-trinitro-2,4,6-trinitroaminobenzene (TNTNB), which was synthesized using an acylation-activation-nitration method. TNTNB exhibits a high density (d: 1.995 g cm−3 at 173 K, 1.964 g cm−3 at 298 K) and excellent heat of detonation (Q: 7179 kJ kg−1), which significantly exceed those of HNB (Q: 6993 kJ kg−1) and the state-of-the-art explosive CL-20 (Q: 6534 kJ kg−1); thus, TNTNB represents the new energy peak for organic explosives. Compared to HNB, TNTNB also exhibits enhanced chemical stability in water, acids, and bases.
摘要 :硝基越多,苯的能量越高,但化学稳定性越低。自1966年以来,具有完全硝化结构的六硝基苯(HNB)一直是有机炸药的能量峰,但其非常不稳定,甚至在潮湿空气中分解。为了提高能量极限,平衡能量和化学稳定性,我们提出了区间全硝基硝氨基协同策略,通过酰化-活化-硝化的方法合成了一种新的全硝化苯,1,3,5-三硝基-2,4,6-三硝氨基苯化合物(TNTNB)。TNTNB具有高密度(d:173 K时密度为1.995 g cm−3,298 K时密度为1.964 g cm−3)和极好的爆热(Q:7179 kJ kg−1),其爆热大大超过HNB(Q:6993 kJ kg−1)和最先进的炸药CL-20(Q:6534 kJ kg−1);因此,TNTNB代表有机炸药的新能量峰值。与HNB相比,TNTNB在水、酸和碱等溶液中也表现出更强的化学稳定性。
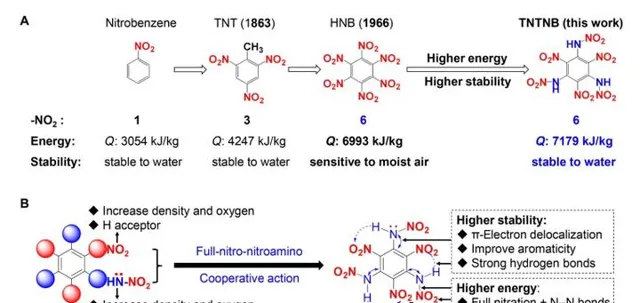
The background of nitro-benzenes and design strategy of TNTNB . (A) Comparison of the number of -NO2 groups, energy (Q: heat of detonation), and stability (chemical stability) of nitrobenzene, TNT, HNB, and TNTNB, respectively. (B) Design of TNTNB through interval full-nitro-nitroamino cooperative action.
硝基苯的背景和TNTNB的设计策略 。(A)分别比较硝基苯、TNT、HNB和TNTNB的-NO2基团数、能量(Q:爆热)和稳定性(化学稳定性)。(B)通过区间全硝基硝氨基协同作用设计TNTNB。
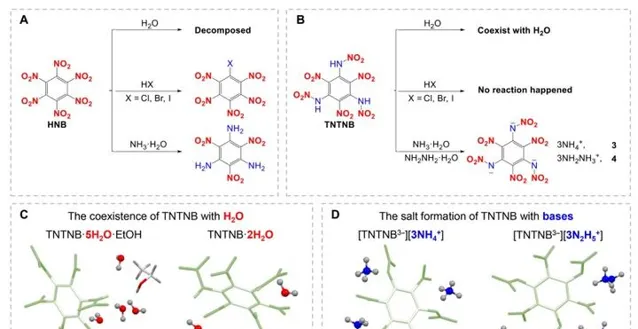
Chemical stability of HNB and TNTNB with water, acids, and bases, respectively . (A) Chemical stability of HNB. (B) Chemical stability of TNTNB. (C) Single-crystal x-ray structural confirmation of chemical stability of TNTNB with pO, namely, TNTNB·5pO·EtOH and TNTNB·2pO. (D) Single-crystal x-ray structural confirmation of chemical stability of TNTNB with bases, namely, [TNTNB3−][3Np+] (compound 3) and [TNTNB3−][3N2p+] (compound 4).
HNB和TNTNB分别与水、酸和碱的化学稳定性 。(A)HNB的化学稳定性。(B)TNTNB的化学稳定性。(C)TNTNB与pO的化学稳定性的单晶X射线结构确认,即TNTNB·5pO·EtOH和TNTNB·2pO。(D)TNTNB与碱的化学稳定性的单晶X射线结构确认,即[TNTNB3−][3Np+](化合物3)和[TNTNB3−][3N2p+](化合物4)。
文献来源:
https://www. science.org/doi/epdf/10 .1126/sciadv.abn3176
以上中文翻译为译者个人对于文章的概略理解,论文传递的准确信息请参照英文原文。
科学足迹出版社
Science Footprint Press, SFP
*长按左侧二维码关注我们 *
或微信搜索公众号「科学足迹」











